Let’s have a look at what causes acid rain. Damaged buildings, dying plants and fish – These are some of the acid rain effects! But do you know what acid rain is? It is rain or any other participates from the atmosphere such as fog, mist, hale or snow that is more acidic than normal
Let’s have a look at what causes acid rain. Damaged buildings, dying plants and fish – These are some of the acid rain effects! But do you know what acid rain is? It is rain or any other participates from the atmosphere such as fog, mist, hale or snow that is more acidic than normal rain fog, mist, hale or snow. The word acid comes from the Latin word “acidus”, that means sour. Vinegar has a sour taste because it is acidic.
How is acid rain formed?
The earth is surrounded by an atmosphere. The atmosphere is the mixture of gasses that surround the earth. Some of the gasses that can be found in the atmosphere are nitrogen and oxygen. Tiny dust particles and pollution gasses also form part of the mixture of gasses and particles in the atmosphere.
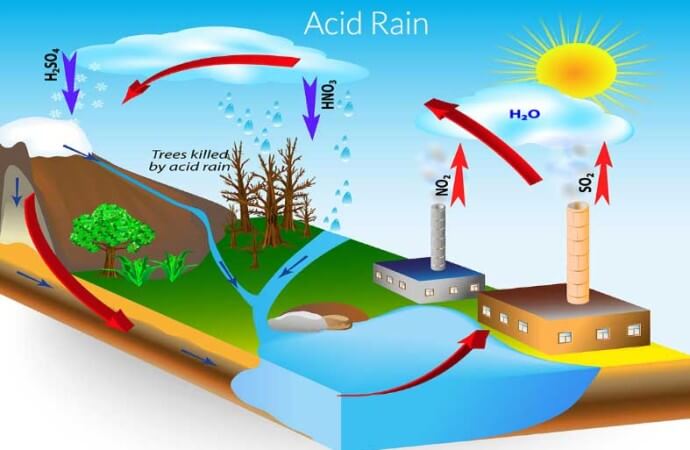
All rainwater or participates from the atmosphere are slightly acidic, but acid rain precipitates are more acidic than normal. So, what causes acid rain? The main culprit is pollution. Pollution gasses are normally released when fossil fuels, such as coal and petroleum, are burned to make energy. Pollution gasses such as sulphur dioxide (SO2) and nitrogen oxides (NOx) are released into the atmosphere and forms part of the process where rain is made. In the air the gasses mix with water vapour (very small water particles floating around like steam from a kettle). When the air cools down, the water vapour is condensed (forms a liquid from a gas) and mist or raindrops containing pollution gasses are formed.
Acid rain definition:
Now let’s look at the more compact definition for acid rain. Acid rain is precipitates that are more acidic than common precipitates from the atmosphere. The raindrops or other precipitates are made acidic by atmospheric pollution, mainly coming from the burning of fossil fuels. The pollution gasses such as sulphur dioxide and nitrogen oxides mix with atmospheric water vapour and acid rain forms. Acid rain causes damage to the environment by harming plants, damaging buildings and cars and by making water and soil more acidic.
How does acid rain affect the environment?
Acid rain causes the ocean, lakes, rivers, and dams to become more acidic. Certain fish species are very sensitive to the water pH (a measure of how acidic or basic (non-acidic) a solution is). Acid rain can cause pH sensitive water animals to die and it can even stop fish eggs from hatching!
Soil can also become more acidic due to acid rain. Some plants will not be able to grow in the soil when it becomes too acidic or they will grow very slowly. When the soil becomes more acidic, too much of certain minerals in the soil can dissolve or become more available to plants. A dangerous example of these minerals is aluminium. Aluminium and other minerals can reach lakes, rivers, dams and the ocean, where it can kill animals that live in the water or it can gather in the animal’s body. When a mineral such as aluminium gathers in an animal’s body, it can kill the animal when too much aluminium has built up over time. The animal with all the aluminium in its body can be eaten by other animals or humans and this can cause illness or death.
Buildings, statues and cars can be damaged by acid rain. Metals can be corroded (weakened or destroyed by a chemical reaction) building material can be weakened and will start to crumble. Even the paint used on buildings and cars can be damaged by the acid in acid rain. All this costs a lot of money because buildings and cars have to be repainted and maintained.
Acid rain can affect human health because the small pollution particles in the acid rain in the atmosphere can cause lung disorders. The particles in the atmosphere can travel great distances when the wind blows really strong and when it rains; these particles are released into the environment. The particles can be inhaled and lung disorders such as asthma can be increased in areas where there is acid rain.
Acid rain facts:
Did you know that although it is mostly human activities that cause acid rain, some acid rain can occur naturally! Sulphur dioxide and nitrogen compounds from volcanoes and rotting plants can cause acid rain to form.
Some water bodies (lakes, dams, rivers, streams, oceans) are not affected as much as other water bodies because limestone (a type of rock) may be near the water body in the soil. Limestone helps to neutralize (not too acidic and not too basic) the acid from acid rain.
Rain that falls on Venus (second planet in our solar system) is made from sulphuric acid. This is extremely acidic rain.
How to prevent acid rain:
We now know that acid rain can cause a lot of damage to our environment. We can help to prevent acid rain by preventing pollution. We can start using “cleaner” technology to create energy such as energy from solar panels or wind turbines and we can reduce the burning of fossil fuels.
What do you think is a great plan to prevent acid rain?













































Leave a Reply