What would happen if you took a piece of anything – gold, for example – and kept cutting it in half and half again, into smaller and smaller pieces? It would make sense to think that at some point you wouldn’t be able to cut it anymore. Then you’d be left with the smallest piece
What would happen if you took a piece of anything – gold, for example – and kept cutting it in half and half again, into smaller and smaller pieces? It would make sense to think that at some point you wouldn’t be able to cut it anymore. Then you’d be left with the smallest piece of gold possible, wouldn’t you?
Democritus Atomic Theory
This was certainly what the very first atomic theory said. Democritus, an ancient Greek philosopher, seems to have been the first person to come up with a theory about atoms that is similar to what modern technology tells us. He said that everything in the world was made up of atoms, and those atoms were the smallest possible parts and couldn’t be broken into smaller parts. He also said that there was space between the atoms where there was nothing – a void. Finally, Democritus’ atomic theory also said that every different material was made of different atoms which had different properties. For example, he thought that stone atoms had little hooks holding them close together, and that’s why stone is solid and hard to break apart. Water atoms were slippery, while air atoms were light and “airy”.
Though atomic theory has changed over time, Democritus’ ideas about atoms were the first to come close to what science has now discovered.
Dalton Atomic Theory
English physicist John Dalton, in the early 1800s, also started thinking about atoms and came up with his own atomic theory. Dalton measured the weights of different compounds and found that chemicals seem to combine in predictable ways. For example, he found that tin and oxygen can combine to produce 2 different oxides (like tin rust) with different weights with one double the other. He guessed that 1 tin atom would combine with either 1 or 2 oxygen atoms. From this, he came up with a theory that every different element is made up of atoms which are all the same for each element, and different from other elements. It was these atoms that combined and separated in chemical reactions. He also believed, like the ancient Greeks, that atoms could not be broken down into smaller pieces.
Rutherford Atomic Theory
Further experiments by physicists changed the way that we think about atoms. One scientist, J.J. Thompson, discovered electrons and correctly believed that these electrically charged particles were present in every atom. He thought electrons were spread out evenly in atoms, like raisins sprinkled over a plum pudding, so this atomic theory was called the plum pudding model.
Physicist Ernest Rutherford, in 1909, did experiments to confirm the plum pudding model and instead ended up with his own atomic theory. He shot alpha particles through a sheet of ultra-thin gold foil. Because the alpha particles were heavy, but the electron “raisins” they expected in the gold atoms were light, he thought the alpha particles would go straight through the foil or just get deflected a little bit by the electrons. But what he found was that some of the alpha particles went through and some were reflected right back the way they had come! This meant that there was something heavier and bigger than the electrons in the atom that could make the alpha particles bounce back.
Rutherford had discovered the nucleus of an atom. This was the heavy center of the atom and Rutherford’s atomic model was like a planet with moons – a heavy nucleus in the middle of each atom with electrons orbiting around it.
Bohr Atomic Theory
Danish scientist Niels Bohr did experiments to show that electrons don’t just orbit around nuclei any way they like. Instead, they orbit in predictable levels, or “shells”, and can jump from one shell up to a higher one when given more energy. This part of atomic theory was followed by the discoveries of bother protons and neutrons in the nucleus of each atom, giving us a much clearer picture of how atoms are actually put together.
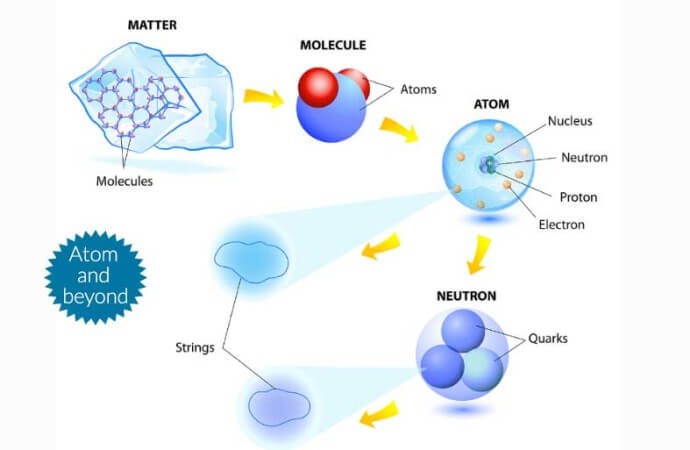
What is an Atom? – A Complete Picture
Thanks to centuries of research, we now know the following about atoms:
- Atoms are constructed of neutral particles (neutrons) and positively charged particles (protons) contained in the nucleus, as well as negatively charged particles (electrons) which orbit the nucleus in set levels.
- Atoms of one element all have the same number of protons. If an atom of an element has fewer or more neutrons than normal for that element, it’s called an isotope and has different characteristics than regular atoms. If an atom has fewer or more electrons than normal, it will be an electrically charged particle called an ion.
- In nature there are 90 different kinds of atoms. Scientists have made or observed another 25 types.
- Atoms of different elements have different numbers of protons, neutrons, and electrons giving them different atomic weights and causing them to behave differently.













































Leave a Reply