Have you ever heard about the term “osmosis”? Let’s find out more about this strange word, so that you can tell your friends all about it! Diffusion and osmosis: Before I can begin to tell you about osmosis, you first have to ask yourself the question: What is diffusion? Diffusion is the random movement of
Have you ever heard about the term “osmosis”? Let’s find out more about this strange word, so that you can tell your friends all about it!
Diffusion and osmosis:
Before I can begin to tell you about osmosis, you first have to ask yourself the question: What is diffusion? Diffusion is the random movement of molecules (liquid, solid or gas molecules) from a region of a higher concentration to a region with a lower concentration. Do you know what it means when we say that something has a higher concentration? It means that there are a lot of molecules together in a certain space, so when something has a lower concentration, there are fewer molecules in a certain space. Let’s look at an example of diffusion. Have you ever placed a few drops of food colouring into a glass of water? If not, why don’t you try it? All the beautiful coloured drops that you have place in a glass of water will start to spread out on its own, until all the water is the same colour. Let’s say that you place red food colouring in the water. The drops will be dark red, but as soon as you drip the drops into the water, the colour will start to get lighter as it spreads out in the glass. This very interesting occurrence is called diffusion. A more formal definition of diffusion state that: Diffusion is when molecules spread out from an area of a high concentration to an area of a low concentration, in order to reach equilibrium (where all the molecules are equally spread out in a space).
Now, let’s look at a complete definition of osmosis and then use fun examples of osmosis so that you can understand it perfectly. We can define osmosis as a process by which molecules of a solvent (a liquid that dissolves other liquids solids or gasses) tend to pass through a semipermeable membrane from a less concentrated solution into a more concentrated one. Did you know that water is a solvent that all of you drink every day? Let’s make sure that all of you know what the words in the definition means. Did you wonder what a semipermeable membrane is? A semipermeable membrane is a type of layer that only lets certain molecules through. It is exactly the same as a sieve! When you throw some fine sand and a few pebbles into a sieve, all the sand will fall through and the pebbles will stay in the sieve.
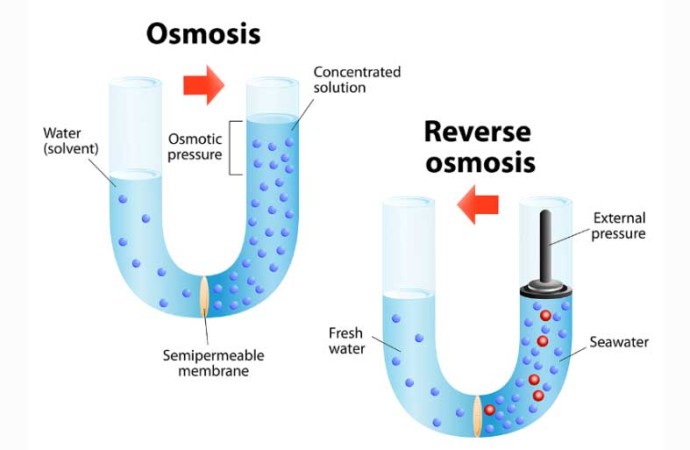
Let’s look at an example of salt and water to explain osmosis a bit better. Imagine that we have a big container with a marked water level and that we put a semipermeable membrane in the container that will divide the whole container into two parts. This semipermeable membrane, that we put in the container, can only let the small water molecules through, but the big salt molecules cannot fit through.
Now, imagine that we throw more salt into the one side of the container than the other, and fill the two parts of the container up with water, so that both parts of the container are filled up to the same level. The salt dissolves in the water when we add the water to both parts of the container. Only the water molecules will start to move through the semipermeable membrane, towards the part of the container that has the most salt molecules (where there are less water molecules). So, the water molecules will move through the semipermeable membrane, from the part of the container with the lower concentration of salt molecules, to the part of the container with the higher concentration of salt molecules.
The water molecules are moving to the other part of the container so that there can be the same amount of water molecules in both parts of the container. We will see the water level start rising in the part of the container that has more of the salt molecules, because all the free water molecules are moving to that part of the container.
Osmosis vs diffusion:
Can you see the difference between osmosis and diffusion yet? Diffusion involves the movement of molecules (gas, liquid or solid molecules) from an area of a high concentration to an area of a lower concentration. Osmosis on the other hand is a type of diffusion, but only a solute (such as water) moves through a semipermeable membrane, from an area with a low concentration of dissolved molecules to an area with a higher concentration of dissolved molecules.
Does osmosis require energy to work? – The Similarity between diffusion and osmosis:
Diffusion and osmosis are both forms of passive transport. Passive transport means that the molecules do not need energy to move from a high concentration to a low concentration, such as in diffusion. The molecules in a solute, such as water, also do not need energy to move through a semipermeable membrane, from a low- to a high concentration of dissolved molecules (osmosis).
Why don’t you watch the following video, where osmosis is explained, so that you can see all the moving molecules: https://www.youtube.com/watch?v=w3_8FSrqc-I
What do you think will happen to a cell with a semipermeable membrane, when the water in the cell has less dissolved molecules than the water in the surrounding environment?













































Leave a Reply